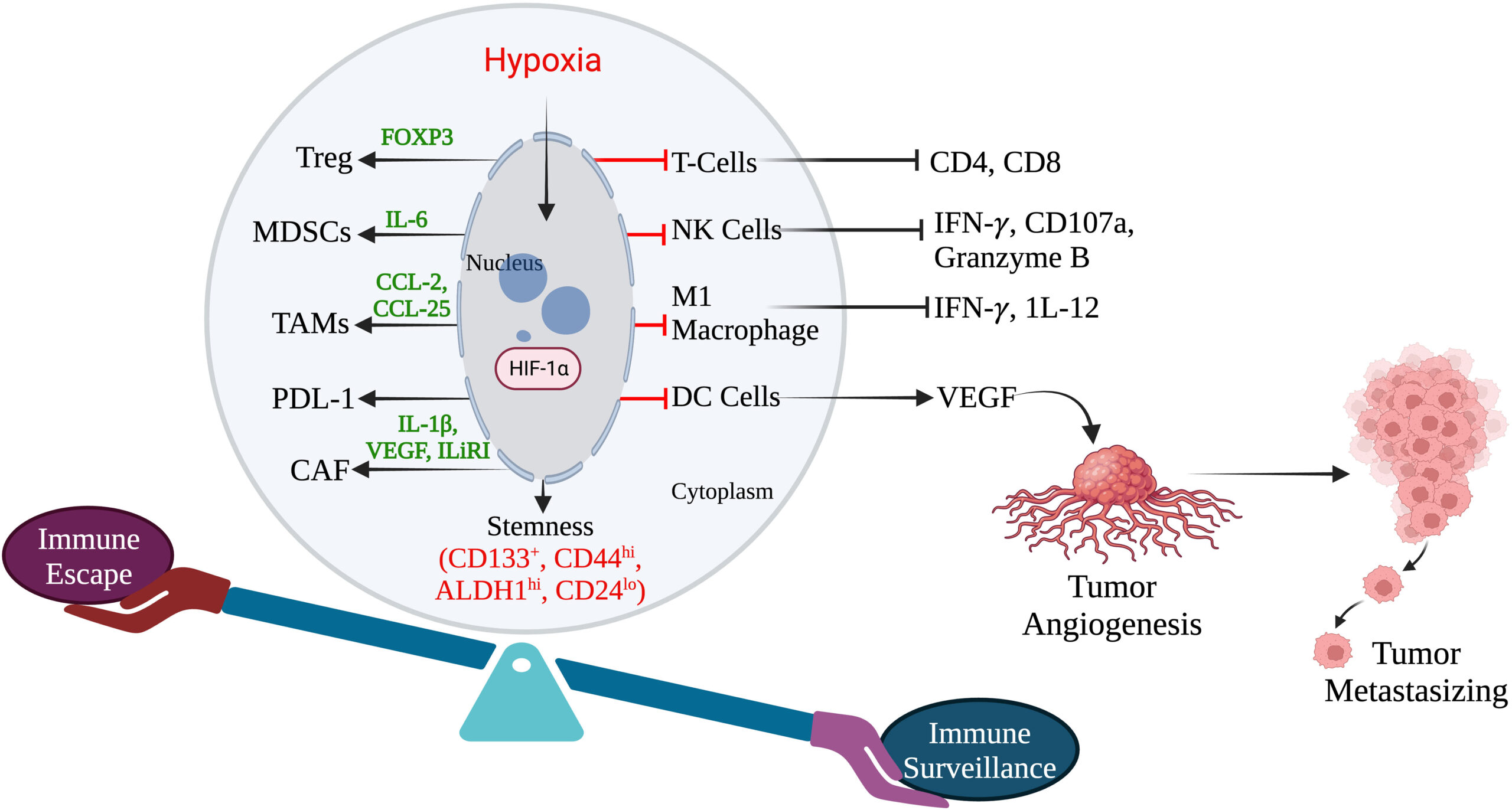
Nityanand S and his group of collaborators from india and USA published an in-depth review meticulously about how hypoxia play lead role in triple negative breast cancer in the frontiers of oncology.
Triple-negative breast cancer (TNBC) is an aggressive form of breast cancer characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) expression. Its treatment poses a significant challenge due to the lack of targeted therapies. Hypoxia, a condition where there is an inadequate oxygen supply to tissues, plays a crucial role in TNBC progression and treatment resistance. In this article, we explore the impact of hypoxia on TNBC and its potential as a therapeutic target.
Understanding Hypoxia in TNBC: Hypoxia is a common feature of solid tumors, including TNBC. It occurs when tumor growth outpaces the blood vessel formation, leading to inadequate oxygen supply. Hypoxia activates various molecular pathways, including hypoxia-inducible factor-1 alpha (HIF-1α), which plays a key role in promoting tumor survival, angiogenesis, metastasis, and resistance to therapy.
Hypoxia-Driven Therapeutic Resistance: Emerging evidence suggests that hypoxia contributes to treatment resistance in TNBC. Hypoxic regions within the tumor microenvironment promote the development of cancer stem cells (CSCs), which are highly resistant to chemotherapy and radiation therapy. Additionally, hypoxia-induced molecular changes alter the tumor’s response to conventional therapies, leading to decreased efficacy.
Targeting Hypoxia for TNBC Treatment: Recognizing the impact of hypoxia on TNBC, researchers are exploring strategies to target hypoxic regions to improve treatment outcomes. Several approaches are being investigated:
Hypoxia-Activated Prodrugs: Hypoxia-activated prodrugs are designed to selectively release cytotoxic agents in hypoxic regions. These prodrugs remain inactive under normal oxygen conditions but become activated in the presence of low oxygen levels. By targeting hypoxic tumor regions, these drugs aim to enhance treatment efficacy while minimizing damage to normal tissues.
Modulating Hypoxia-Inducible Factor (HIF) Pathways: Targeting HIF pathways, including HIF-1α, is another approach to disrupt hypoxia-mediated tumor progression. Researchers are exploring small molecules and inhibitors that can modulate HIF pathways, thereby reducing angiogenesis, inhibiting metastasis, and enhancing treatment sensitivity.
Combination Therapies: Combining hypoxia-targeting agents with conventional therapies holds promise for overcoming treatment resistance in TNBC. By simultaneously targeting hypoxic regions and tumor cells, combination therapies aim to enhance treatment response and reduce the likelihood of relapse.
Clinical Trials and Future Perspectives: Numerous clinical trials are underway to evaluate the effectiveness of hypoxia-targeted therapies in TNBC. These trials aim to determine the safety, efficacy, and optimal combinations of hypoxia-targeting agents with standard treatments. Continued research and advancements in understanding the complex interplay between hypoxia and TNBC will pave the way for innovative therapeutic approaches.
Conclusion: Hypoxia represents a significant challenge in the treatment of triple-negative breast cancer. Its association with treatment resistance and poor prognosis necessitates the development of targeted strategies. Targeting hypoxic regions through hypoxia-activated prodrugs, modulating HIF pathways, and exploring combination therapies offer potential avenues to improve treatment outcomes for TNBC patients. Through ongoing research and clinical trials, we aim to overcome the challenges posed by hypoxia and provide effective therapies that can combat TNBC and improve patient outcomes.
Source:https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2023.1199105/full